AB CD CB AD In the given representation A has switched position with C and similarly C with A. A double displacement reaction can be represented as follows.

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com
A double replacement reaction is a type of chemical reaction in which two reagents exchange ions to form two new products with the same type of chemical bonds.

. The chemical bonds between the reactants ma. In this type of reaction the positive-charged cations and the negative-charged anions of the reactants both trade places double displacement to form two new products. You can predict whether a single-displacement reaction will occur by comparing the reactivity of an element using an activity series table.
Many double displacement reactions occur between ionic compounds that are dissolved in water. This ScienceStruck post explains the concept of double displacement reaction in chemistry along with a few examples. A chemical reaction between two compounds in which the first and second parts of one reactant are united respectively with the second and first parts.
While some reactions involve a change in the state of matter eg liquid to gas phase a phase change is not necessarily an indicator of a reaction. An example is the reaction between silver nitrate and sodium chloride to form silver chloride and sodium nitrate. Double displacement reaction are those in which two chemicals react by exchanging ions to produce two new molecules.
A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products. It All Depends on the Resulting Product. A double replacement reaction is a type of chemical reaction where two reactants exchange ions to form two new products with the same type of chemical bonds.
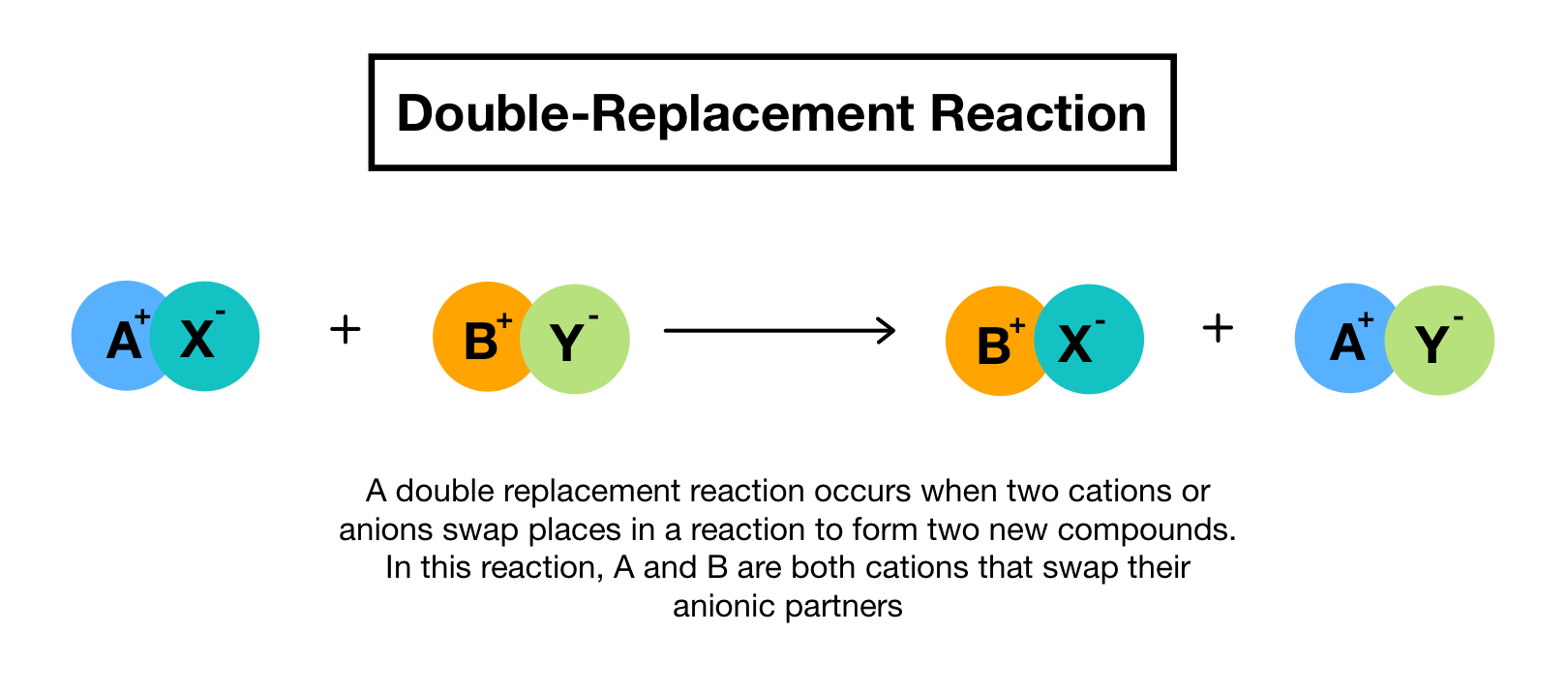
The cations and anions of two compounds switch places to form two new products in a double replacement or double displacement reaction. Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. Double replacement reactions are also known as double displacement exchange or metathesis processes.
A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. Create your own flashcards or choose from millions created by other students. Give double displacement reaction examples.
Ionic chemicals dissolved in water undergo a lot of double displacement processes. Usually a double displacement reaction results in precipitate formation. Double displacement reaction definition.
A double displacement reaction is a type of chemical reaction where two compounds react and positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. AB CD AD CB. Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds.
A double replacement reaction is. Double displacement reactions are those in which the reactant compounds exchange positive ions with each other to form new compounds. Double Displacement or Replacement Reaction - Also called a metathesis reaction this occurs when both cations and anions of the reactants trade places to form products.
The overall pattern of a double replacement reaction looks like this. B a C l 2 a q N a 2 S O 4 a q B a S O 4 s 2 N a C l a q Combustion Reactions. Double displacement synonyms double displacement pronunciation double displacement translation English dictionary definition of double displacement.
A double displacement reaction takes place when two iconic components of a compound switch positions with each other. The joints and anonyms of two compounds change places to form two new products in a double replacement or double displacement reaction. Double displacement reactions are reactions where the cations and anions in the reactants switch partners to form products.
Usually one of the products forms a. Quizlet is the easiest way to study practice and master what youre learning. AgNO 3 NaCl AgCl NaNO 3.
For example Na 2 S2HCl2NaClH 2 S REVISE WITH CONCEPTS Combination Reaction Example Definitions Formulaes. Other names for a double displacement reaction are a metathesis reaction or a double replacement reaction. In double replacement reactions the positive ions exchange negative ion partners.
Positive ions exchange negative ion partners in double replacement processes. In general a metal can displace any metal lower in the activity series cations. Double-Replacement Reactions When components of two ionic compounds are swapped two new compounds are formed.
Usually when two compounds react both cations or both anions will change partners producing a double-displacement reaction. A displacement reaction occurs when a more reactive element displaces or pushes out a less reactive element from a compound that contains the less reactive element. Examples of double displacement.
A double displacement reaction is an important type of chemical reaction to know when studying chemistry. This quizworksheet will help you test.
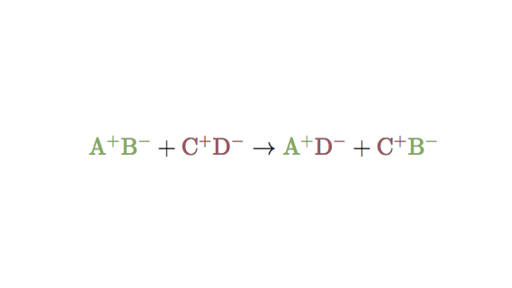
Double Replacement Reactions Double Displacement Article Khan Academy

Single Replacement Reaction Definition And Examples
Double Replacement Reactions Definition Examples Expii

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Double Replacement Double Displacement Reaction

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube


0 komentar
Posting Komentar